Atomic Number 10 Atomic Number 10 is belong to element of Neon. Chemical symbol for Neon is Ne. Number of protons in Neon is 10. Atomic weight of Neon is 20.1797 u or g/mol. Each element is identified by the number of protons in its atoms. This number is the atomic number. The periodic table lists the elements in order of increasing atomic number. Each element has a symbol, which is one or two letters. The first letter is always capitalized. If there is a second letter, it is lowercase. Atomic mass: The mass of an atom is primarily determined by the number of protons and neutrons in its nucleus. Atomic mass is measured in Atomic Mass Units (amu) which are scaled relative to carbon, 12 C, that is taken as a standard element with an atomic mass of 12.
Now you know that atomic number = number of protons, and mass number = number of protons + number of neutrons. To find the number of neutrons in an element, subtract the atomic number from the mass number. Here are a couple example: A single helium (He) atom has a mass number of 4 and an atomic number of 2. It must have 4 - 2 = 2 neutrons. Atomic Number 10 Atomic Number 10 is belong to element of Neon. Chemical symbol for Neon is Ne. Number of protons in Neon is 10. Atomic weight of Neon is 20.1797 u or g/mol.
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
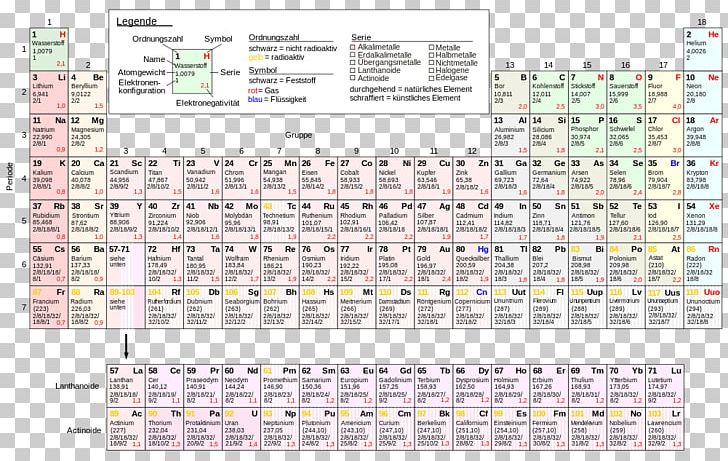
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
The elements of the periodic table sorted by atomic number
click on any elements name for further chemical properties, environmental data or health effects.
This list contains the 118 elements of chemistry.
| The chemical elements of the periodic chart sorted by: | Atomic number | Name chemical element Apple music recognition. Nov 20, 2020 IOS 14.2 includes a new music recognition feature. It builds Shazam, which Apple acquired in 2018, right into your iPhone. You can find out what song is playing with just a tap. Here's how to set. Plus your entire music library on all your devices. Sep 28, 2020 Music Recognition works with songs playing in the ambient environment as well as songs playing on your iPhone. So if you're watching a video and want to know what song is in it, you can just tap. | Symbol |
| - Name alphabetically | 1 | Hydrogen | H |
| - Atomic number | 2 | Helium | He |
| - Symbol | 3 | Lithium | Li |
| - Atomic Mass | 4 | Beryllium | Be |
| - Electronegativity | 5 | Boron | B |
| - Density | 6 | Carbon | C |
| - Melting point | 7 | Nitrogen | N |
| - Boiling point | 8 | Oxygen | O |
| - Vanderwaals radius | 9 | Fluorine | F |
| - Year of discovery | 10 | Neon | Ne |
| - Inventor surname | 11 | Sodium | Na |
| - Elements in earthcrust | 12 | Magnesium | Mg |
| - Elements in human body | 13 | Aluminum | Al |
| - Covalenz radius | 14 | Silicon | Si |
| - Ionization energy | 15 | Phosphorus | P |
For chemistry students and teachers: The tabular chart on the right is arranged by Atomic number. The first chemical element is Hydrogen and the last is Ununoctium. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | 16 | Sulfur | S |
| 17 | Chlorine | Cl | |
| 18 | Argon | Ar | |
| 19 | Potassium | K | |
| 20 | Calcium | Ca | |
| 21 | Scandium | Sc | |
| 22 | Titanium | Ti | |
| 23 | Vanadium | V | |
| 24 | Chromium | Cr | |
| 25 | Manganese | Mn | |
| 26 | Iron | Fe | |
| 27 | Cobalt | Co | |
| 28 | Nickel | Ni | |
| 29 | Copper | Cu | |
| 30 | Zinc | Zn | |
| 31 | Gallium | Ga | |
| 32 | Germanium | Ge | |
| 33 | Arsenic | As | |
| 34 | Selenium | Se | |
| 35 | Bromine | Br | |
| 36 | Krypton | Kr | |
| 37 | Rubidium | Rb | |
| 38 | Strontium | Sr | |
| 39 | Yttrium | Y | |
| 40 | Zirconium | Zr | |
| 41 | Niobium | Nb | |
| 42 | Molybdenum | Mo | |
| 43 | Technetium | Tc | |
| 44 | Ruthenium | Ru | |
| 45 | Rhodium | Rh | |
| 46 | Palladium | Pd | |
| 47 | Silver | Ag | |
| 48 | Cadmium | Cd | |
| 49 | Indium | In | |
| 50 | Tin | Sn | |
| 51 | Antimony | Sb | |
| 52 | Tellurium | Te | |
| 53 | Iodine | I | |
| 54 | Xenon | Xe | |
| 55 | Cesium | Cs | |
| 56 | Barium | Ba | |
| 57 | Lanthanum | La | |
| 58 | Cerium | Ce | |
| 59 | Praseodymium | Pr | |
| 60 | Neodymium | Nd | |
| 61 | Promethium | Pm | |
| 62 | Samarium | Sm | |
| 63 | Europium | Eu | |
| 64 | Gadolinium | Gd | |
| 65 | Terbium | Tb | |
| 66 | Dysprosium | Dy | |
| 67 | Holmium | Ho | |
| 68 | Erbium | Er | |
| 69 | Thulium | Tm | |
| 70 | Ytterbium | Yb | |
| 71 | Lutetium | Lu | |
| 72 | Hafnium | Hf | |
| 73 | Tantalum | Ta | |
| 74 | Tungsten | W | |
| 75 | Rhenium | Re | |
| 76 | Osmium | Os | |
| 77 | Iridium | Ir | |
| 78 | Platinum | Pt | |
| 79 | Gold | Au | |
| 80 | Mercury | Hg | |
| 81 | Thallium | Tl | |
| 82 | Lead | Pb | |
| 83 | Bismuth | Bi | |
| 84 | Polonium | Po | |
| 85 | Astatine | At | |
| 86 | Radon | Rn | |
| 87 | Francium | Fr | |
| 88 | Radium | Ra | |
| 89 | Actinium | Ac | |
| 90 | Thorium | Th | |
| 91 | Protactinium | Pa | |
| 92 | Uranium | U | |
| 93 | Neptunium | Np | |
| 94 | Plutonium | Pu | |
| 95 | Americium | Am | |
| 96 | Curium | Cm | |
| 97 | Berkelium | Bk | |
| 98 | Californium | Cf | |
| 99 | Einsteinium | Es | |
| 100 | Fermium | Fm | |
| 101 | Mendelevium | Md | |
| 102 | Nobelium | No | |
| 103 | Lawrencium | Lr | |
| 104 | Rutherfordium | Rf | |
| 105 | Dubnium | Db | |
| 106 | Seaborgium | Sg | |
| 107 | Bohrium | Bh | |
| 108 | Hassium | Hs | |
| 109 | Meitnerium | Mt | |
| 110 | Darmstadtium | Ds | |
| 111 | Roentgenium | Rg | |
| 112 | Copernicium | Cn | |
| 113 | Nihonium | Nh | |
| 114 | Flerovium | Fl | |
| 115 | Moscovium | Mc | |
| 116 | Livermorium | Lv | |
| 117 | Tennessine | Ts | |
| 118 | Oganesson | Og |

| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
The elements of the periodic table sorted by atomic number
click on any elements name for further chemical properties, environmental data or health effects.
This list contains the 118 elements of chemistry.
| The chemical elements of the periodic chart sorted by: | Atomic number | Name chemical element Apple music recognition. Nov 20, 2020 IOS 14.2 includes a new music recognition feature. It builds Shazam, which Apple acquired in 2018, right into your iPhone. You can find out what song is playing with just a tap. Here's how to set. Plus your entire music library on all your devices. Sep 28, 2020 Music Recognition works with songs playing in the ambient environment as well as songs playing on your iPhone. So if you're watching a video and want to know what song is in it, you can just tap. | Symbol |
| - Name alphabetically | 1 | Hydrogen | H |
| - Atomic number | 2 | Helium | He |
| - Symbol | 3 | Lithium | Li |
| - Atomic Mass | 4 | Beryllium | Be |
| - Electronegativity | 5 | Boron | B |
| - Density | 6 | Carbon | C |
| - Melting point | 7 | Nitrogen | N |
| - Boiling point | 8 | Oxygen | O |
| - Vanderwaals radius | 9 | Fluorine | F |
| - Year of discovery | 10 | Neon | Ne |
| - Inventor surname | 11 | Sodium | Na |
| - Elements in earthcrust | 12 | Magnesium | Mg |
| - Elements in human body | 13 | Aluminum | Al |
| - Covalenz radius | 14 | Silicon | Si |
| - Ionization energy | 15 | Phosphorus | P |
For chemistry students and teachers: The tabular chart on the right is arranged by Atomic number. The first chemical element is Hydrogen and the last is Ununoctium. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | 16 | Sulfur | S |
| 17 | Chlorine | Cl | |
| 18 | Argon | Ar | |
| 19 | Potassium | K | |
| 20 | Calcium | Ca | |
| 21 | Scandium | Sc | |
| 22 | Titanium | Ti | |
| 23 | Vanadium | V | |
| 24 | Chromium | Cr | |
| 25 | Manganese | Mn | |
| 26 | Iron | Fe | |
| 27 | Cobalt | Co | |
| 28 | Nickel | Ni | |
| 29 | Copper | Cu | |
| 30 | Zinc | Zn | |
| 31 | Gallium | Ga | |
| 32 | Germanium | Ge | |
| 33 | Arsenic | As | |
| 34 | Selenium | Se | |
| 35 | Bromine | Br | |
| 36 | Krypton | Kr | |
| 37 | Rubidium | Rb | |
| 38 | Strontium | Sr | |
| 39 | Yttrium | Y | |
| 40 | Zirconium | Zr | |
| 41 | Niobium | Nb | |
| 42 | Molybdenum | Mo | |
| 43 | Technetium | Tc | |
| 44 | Ruthenium | Ru | |
| 45 | Rhodium | Rh | |
| 46 | Palladium | Pd | |
| 47 | Silver | Ag | |
| 48 | Cadmium | Cd | |
| 49 | Indium | In | |
| 50 | Tin | Sn | |
| 51 | Antimony | Sb | |
| 52 | Tellurium | Te | |
| 53 | Iodine | I | |
| 54 | Xenon | Xe | |
| 55 | Cesium | Cs | |
| 56 | Barium | Ba | |
| 57 | Lanthanum | La | |
| 58 | Cerium | Ce | |
| 59 | Praseodymium | Pr | |
| 60 | Neodymium | Nd | |
| 61 | Promethium | Pm | |
| 62 | Samarium | Sm | |
| 63 | Europium | Eu | |
| 64 | Gadolinium | Gd | |
| 65 | Terbium | Tb | |
| 66 | Dysprosium | Dy | |
| 67 | Holmium | Ho | |
| 68 | Erbium | Er | |
| 69 | Thulium | Tm | |
| 70 | Ytterbium | Yb | |
| 71 | Lutetium | Lu | |
| 72 | Hafnium | Hf | |
| 73 | Tantalum | Ta | |
| 74 | Tungsten | W | |
| 75 | Rhenium | Re | |
| 76 | Osmium | Os | |
| 77 | Iridium | Ir | |
| 78 | Platinum | Pt | |
| 79 | Gold | Au | |
| 80 | Mercury | Hg | |
| 81 | Thallium | Tl | |
| 82 | Lead | Pb | |
| 83 | Bismuth | Bi | |
| 84 | Polonium | Po | |
| 85 | Astatine | At | |
| 86 | Radon | Rn | |
| 87 | Francium | Fr | |
| 88 | Radium | Ra | |
| 89 | Actinium | Ac | |
| 90 | Thorium | Th | |
| 91 | Protactinium | Pa | |
| 92 | Uranium | U | |
| 93 | Neptunium | Np | |
| 94 | Plutonium | Pu | |
| 95 | Americium | Am | |
| 96 | Curium | Cm | |
| 97 | Berkelium | Bk | |
| 98 | Californium | Cf | |
| 99 | Einsteinium | Es | |
| 100 | Fermium | Fm | |
| 101 | Mendelevium | Md | |
| 102 | Nobelium | No | |
| 103 | Lawrencium | Lr | |
| 104 | Rutherfordium | Rf | |
| 105 | Dubnium | Db | |
| 106 | Seaborgium | Sg | |
| 107 | Bohrium | Bh | |
| 108 | Hassium | Hs | |
| 109 | Meitnerium | Mt | |
| 110 | Darmstadtium | Ds | |
| 111 | Roentgenium | Rg | |
| 112 | Copernicium | Cn | |
| 113 | Nihonium | Nh | |
| 114 | Flerovium | Fl | |
| 115 | Moscovium | Mc | |
| 116 | Livermorium | Lv | |
| 117 | Tennessine | Ts | |
| 118 | Oganesson | Og |
Click here: for a schematic overview of the periodic table of elements in chart form
Do you need to know the weight of some molecules? Try our Molecular Weight Calculator!
Please report any accidental mistake in the above statistics on chemical elements
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Printable List Of Element Names
Copyright © 1998-2021 Lenntech B.V. All rights reserved

